Cell adhesion and cell migration play important roles in variety of physiological and pathological events such as blood vessel formation and remodeling, cancer invasion and metastasis, and inflammation. By using integrins and their associated tetraspanins as example, we are studying the roles and mechanisms of cell adhesion and cell migration in vascular morphogenesis, with emphasis on pathological angiogenesis, and vascular inflammation, with emphasis on vascular stability and leakage. We combine both in vitro and in vivo approaches and integrate experimental and systems biology approaches in our research.
For the research in vascular biology field, our earlier study revealed that tetraspanin CD151 promotes vascular stability, and our recent observations with endothelium-specific Cd151 knockout mice in several vascular hyper-permeability models further support the conclusion of CD151 as enhancer of vascular stability. By balancing Rac1 and RhoA activities, CD151 reinforces endothelial cell-cell and cell-matrix adhesiveness and confines actin cytoskeletal traction to stabilize micro-vasculature and reduce vascular hyper-permeability. The vascular function of tetraspanin CD82 was basically unknown. Our recent study demonstrated that CD82 inhibits pathological angiogenesis in vivo and in vitro. CD82 appears not to alter cell proliferation and survival but inhibits endothelial cell movement, given the fact that major changes of CD82-absent endothelial cells are the enhanced migration and invasion. Such changes in endothelial cell motility are directly linked to the altered endothelial cell-matrix adhesiveness. Together, our study indicates that CD82 and CD151 are needed for properly maintaining the adhesiveness of endothelial cells and coordinately regulate the movement of endothelial cells.
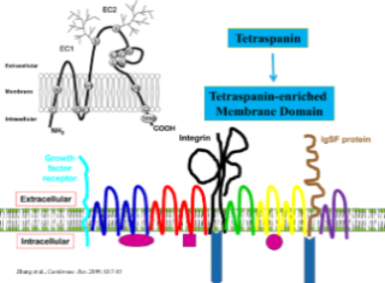
Another research direction of the lab is to understand the role and mechanism of integrins and their associated tetraspanins in tumor progression. Tetraspanins either promote or suppress tumor invasion and metastasis. Their effects on tumor progression depend on the multimolecular transmembrane complex called tetraspanin-enriched membrane domain (TEMD) and are attributed to the alterations in the 1) motogenic and mitogenic behaviors and/or 2) microenvironmental interactions of tumor cells. As the modifiers of cell membrane structure and function, tetraspanins have emerged as diagnostic and prognostic markers and therapeutic targets for tumor progression. Again, our studies centers on tetraspanins CD82 and CD151, which are structurally similar but functionally opposite. Specifically, during tumor progression, CD82 inhibits tumor cell migration and invasion and suppresses tumor metastasis while CD151 promotes tumor cell migration and invasion and facilitates tumor metastasis. For example, we recently demonstrated that, at the molecular level, i) the interaction of CD82 with membrane microdomain is needed for the motility-inhibitory activity of CD82 and ii) the post-translational modifications of CD82 proteins are required for this interaction. At cellular level, the forced expression of CD82 in invasive and metastatic cancer cells results in i) coalescence of TEMD with lipid rafts, ii) attenuation of both growth factor and integrin signaling, and iii) diminution of motility-related events such as protrusion and retraction. Moreover, at the organism level, CD82 suppresses the metastatic steps before and/or at the tumor intravasation. In summary, our study underlines that tetraspanins regulates tumor cell migration, cancer invasion, and cancer metastasis as an organizer for membrane microdomains such as lipid rafts and TEMDs and that tetraspanins are the ideal diagnostic markers and therapeutic target against tumor progression.