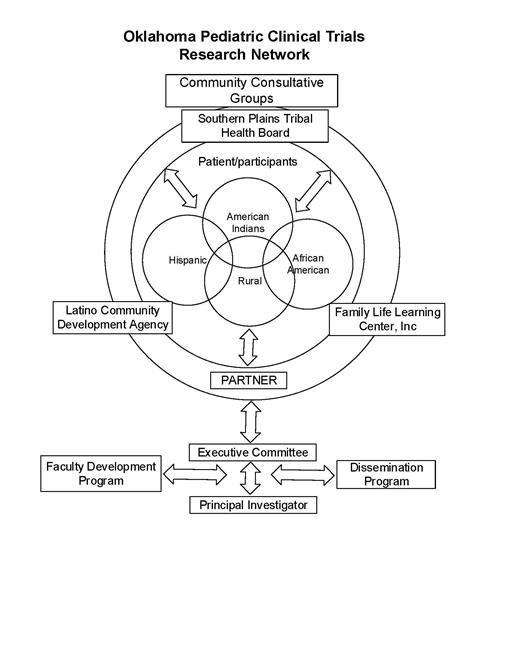
The goal of the Oklahoma Pediatric Clinical Trials Network (OPCTN) is to
provide access for the underserved rural urban minority, and American
Indian populations of children in Oklahoma to state-of-the-art clinical
trials and the transfer of findings that will benefit the health of
those children. The OPCTN will be a member of the IDeA States Pediatric
Clinical Trials Network (ISPCTN). The ISPCTN will facilitate the
involvement of sites in IDeA states to participate in the Environmental
influences on Child Health Outcomes (ECHO) study will focus on four
primary ears of child health: 1) upper and lower airway disease, 2)
obesity; 3) pre-, peri-, and postnatal outcomes; and 4)
neurodevelopment. These are each areas of substantial health concern for
Oklahoma.